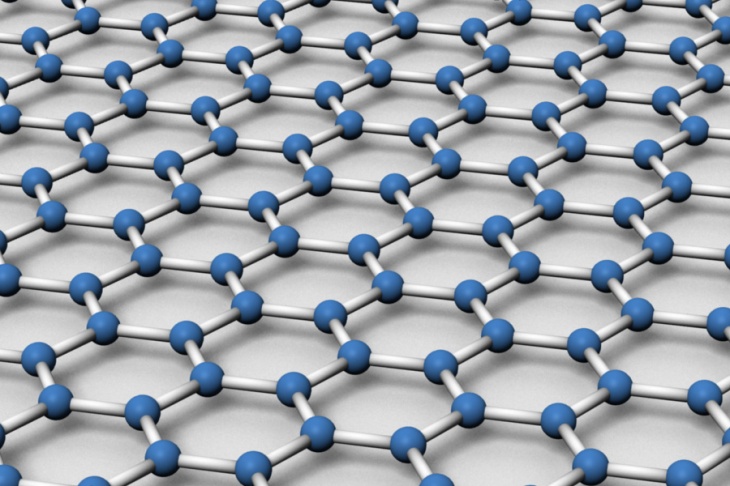
Graphene is an allotrope of carbon in the form of a two-dimensional, atomic scale, honeycomb lattice in which one atom forms each vertex. Following are some of its special properties:
1. It is 100 times stronger than strongest steel.
2. Graphene conducts heat and electricity efficiently.
3. It is the thinnest material possible.
Future applications
1. Sensors
Graphene is an ideal material for sensors. Every atom in graphene is exposed to its environment allowing it to sense changes in surrounding. For chemical sensors, the goal is to be able to detect just one molecule of the potentially dangerous substance. Graphene now allows for the creation of micrometre size sensors capable of detecting individual events on a molecular level.
2. Electronics
Graphene can bring revolutionary changes in the field of electronics. It can give us faster transistors and bendable semiconductors. Graphene could make smartphones which can be rolled like a newspaper.
At just one atom thick and with the ability to conduct electricity at room temperature, graphene semiconductors could replace existing technology for computer chips.